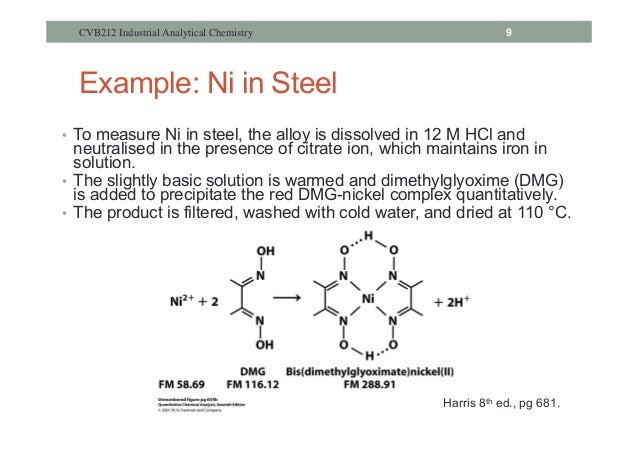
DMG forms a chelating complex with the metal ion and forms a bright red precipitate Ni(C4H7N2O2)2 in a slightly basic solution of 1:1 aqueous ammonia. The precipitate is washed with a 30% ethanol solution and weighed (constant weight) after drying the frits in the oven at 110EC for 2 hours. Gravimetric analysis is one of the most accurate. If a high quantity of these ions is present, a greater amount of DMG must be added. The nickel dimethylglyoximate is a precipitate that is very bulky in character. Therefore, the sample weight used in the analysis must be carefully controlled to allow more convenient.
Summary
A detailed investigation has been made of the gravimetric determination of nickel with dimethylglyoxime in the presence of copper. Copper interference is eliminated by precipitation in a solution containing tartrate and thiosulphate at a pH of 5.5–6.5. The solubility of the nickel dimethylglyoxime complex has been determined as a function of temperature, pH and alcohol concentration.

This is a preview of subscription content, log in to check access.
Access options
Buy single article
34,95 €
Use Of Dmg In Gravimetric Analysis Pdf
Price includes VAT for Germany